For most drivers, starting the car is as simple as turning a key or pressing a button. But behind that effortless action is a powerful chemical and electrical process happening inside your car battery. From delivering an intense burst of energy to powering your vehicle’s electronics, the battery is the silent workhorse that keeps your car going. Let’s take a closer look at what’s happening under the hood and inside the battery the moment you start the engine.
The First Job: Powering the Starter Motor
When you start your car, the battery’s first role is to supply electricity to the starter motor: a small but powerful electric motor that cranks the engine until it can run on its own.
Here’s what happens:
- The starter motor engages a small gear with the engine’s flywheel.
- This gear turns the flywheel, which in turn rotates the crankshaft.
- As the crankshaft turns, the engine’s pistons move and fuel combustion begins. Then, the engine starts running independently.
The starter motor requires a substantial amount of power to perform this task for a brief period. This surge temporarily drains the battery’s stored energy, which is why the battery needs to be recharged afterwards.
How the Alternator Steps In
Once the engine starts, the alternator, a belt-driven generator, begins to rotate. The alternator has two important tasks:
- Recharge the battery by supplying it with electrical energy to replace what was used during starting.
- Power the car’s electronics, such as headlights, infotainment systems and air conditioning.
If your electrical demand exceeds the alternator’s capacity, the battery temporarily supplies the extra energy. But if the engine is switched off, the alternator stops. The battery must power everything until it’s drained to a point where the car will need a jump-start.
Inside the Battery: The Main Components
A typical car battery is a lead-acid battery, usually located in the engine bay. Its key parts include:
- Plastic Case: Holds all internal components securely.
- Terminal Posts: The positive (+) and negative (–) connections for your vehicle’s electrical system.
- Six Internal Cells: Each cell generates about 2.1 volts of direct current (DC). Together, they provide about 12.6 volts when fully charged.
- Lead Plates: Positive plates (cathodes) are made of lead oxide; negative plates (anodes) are made of pure lead.
- Separators: Porous envelopes that keep the plates from touching while allowing ion flow.
- Electrolyte: A mixture of sulfuric acid and water that enables the chemical reaction needed to produce electricity.
Each cell contains both positive and negative plates arranged alternately, with separators between them. The plates are coated with a paste of lead oxide or lead, which acts like a sponge to absorb the electrolyte and improve performance.
The Chemistry Behind the Power
At its core, a car battery works through a reversible chemical reaction between lead, lead oxide and sulfuric acid.
In a charged battery:
- The positive plate (lead oxide, PbO₂) reacts with sulfate ions (SO₄²⁻) from the electrolyte to form lead sulfate (PbSO₄).
- The negative plate (pure lead, Pb) also reacts with sulfate ions to form lead sulfate, releasing electrons in the process.
- These electrons build up on the negative terminal, creating a difference in electrical potential between the two terminals.
When you connect a circuit, like the starter motor, electrons flow from the negative terminal through the circuit to the positive terminal, powering the device along the way.
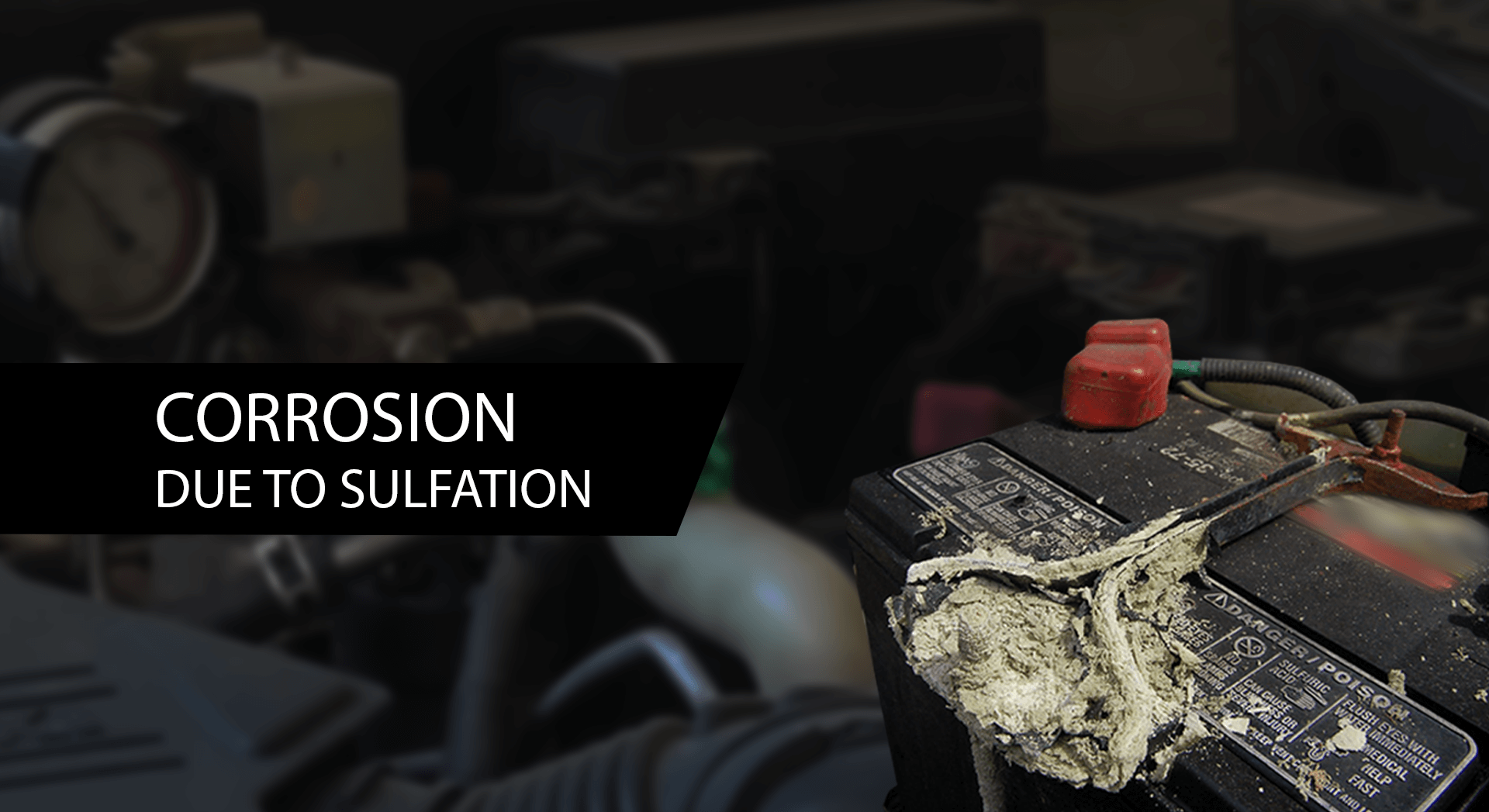
Why the Reaction Doesn’t Last Forever
As the battery discharges:
- The sulfuric acid becomes more diluted as sulfate ions leave the electrolyte and form lead sulfate on the plates.
- A thick coating of lead sulfate builds up, making it harder for the reaction to continue.
Eventually, if left discharged for too long, this lead sulfate can harden, making it difficult to reverse the reaction even when recharging. This is one reason why letting a car battery go flat repeatedly can shorten its lifespan.
Recharging: Running the Reaction in Reverse
When the alternator sends electricity back into the battery:
- Electrons enter the negative terminal and combine with the lead sulfate, returning it to pure lead and releasing sulfate ions back into the electrolyte.
- On the positive plate, the reaction restores lead oxide while also strengthening the electrolyte’s acid concentration.
This reverse process recharges the battery and prepares it for the next engine start.
Testing the Battery’s Health
You can check your car battery using a multimeter:
- Engine off: A healthy, fully charged battery should read about 12.6 volts. A reading below 12 volts suggests it’s not functioning properly.
- Starting: Voltage will drop briefly. If it falls below about 10 volts, the battery may be weak.
- Engine running: The alternator should raise the voltage to around 14 volts to recharge the battery effectively.
The Whole Process in a Few Seconds
When you start your car, here’s the rapid sequence of events:
- Battery sends a surge of current to the starter motor.
- Starter motor turns the engine’s flywheel and crankshaft.
- The engine starts running and fuel combustion begins.
- Alternator starts spinning, recharging the battery and powering electrical systems.
From that point on, as long as the engine runs, the alternator and battery work together to supply the car’s electrical needs.
Why Understanding This Matters
Knowing what happens inside your car battery when you start the engine helps you recognise early signs of trouble, such as slow cranking, dim lights or frequent jump-starts. It also explains why regular driving (which allows the alternator to recharge the battery) is essential to battery health, especially if you use power-hungry accessories.
A car battery may look like a simple black box but without it, your morning drive wouldn’t even begin. Tata Green Batteries are designed with this precision in mind built to provide reliable performance, long life and the confidence that your vehicle will start every time.