From smartphones to electric vehicles, batteries power much of our modern world. But how do these compact devices store energy and deliver it when we need it? To understand that, we need to dive into the basic science of batteries, how they work and why different types perform so differently.
What Is a Battery?
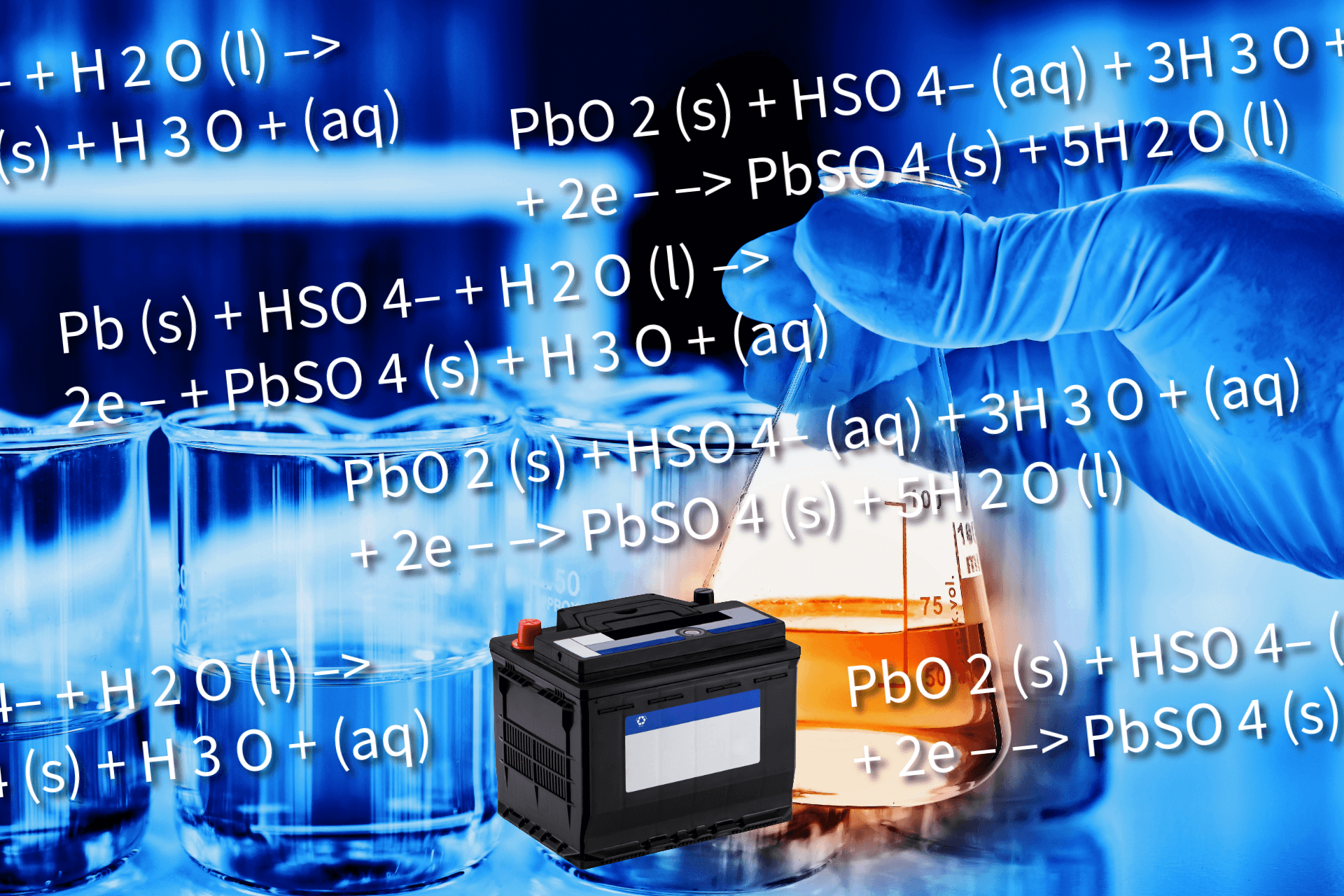
A battery is a device that stores chemical energy and converts it into electrical energy. This is a type of chemical reaction known as a redox reaction or a reduction-oxidation reaction.
A battery is made up of one or more electrochemical cells. Each cell contains three main parts:
- Anode: the negative electrode
- Cathode: the positive electrode
- Electrolyte: a medium that allows ions to flow between the anode and cathode
When the battery is connected to a device, the redox reaction begins. Electrons flow from the anode through the external circuit to the cathode, generating electricity along the way. Meanwhile, ions move through the electrolyte to balance the charge.
How Do Batteries Generate Electricity?
Let’s break down what’s happening here.
-
Oxidation at the Anode
At the anode, atoms lose electrons in a process called oxidation. These electrons are pushed out into the external circuit, which generates the electric current.
-
Electron Flow Through the Circuit
The electrons travel through your device, power your device and eventually reach the cathode.
-
Reduction at the Cathode
At the cathode, another chemical reaction takes place. The atoms there gain electrons; this is the reduction half of the redox pair.
-
Ion Movement in the Electrolyte
To keep the charge balanced, ions move through the electrolyte inside the battery travelling from the anode side to the cathode side.
Together, these reactions allow energy stored in chemical bonds to be released as electrical power.
Rechargeable vs. Non-Rechargeable Batteries
Some batteries are single-use (like alkaline AA batteries), while others can be recharged hundreds or even thousands of times (like the lithium-ion battery in your laptop or EV).
– Primary Batteries: These are non-rechargeable. Once the chemicals are used up, the battery is dead.
– Secondary Batteries: These are rechargeable. The redox reactions can be reversed by applying electrical energy, restoring the original chemical state.
A car battery or a lead acid battery is an example of secondary batteries as the car’s alternator recharges it once the engine is running.
In a lithium-ion battery, for example, charging moves lithium ions back to the anode (usually made of graphite), where they’re stored until needed again. This reversibility is why lithium-ion batteries are widely used in phones or Electric vehicles.
Key Battery Terms You Should Know
Understanding how batteries work also means knowing how they’re measured and compared. Here are a few essential terms:
- Voltage: The electrical pressure that drives electrons through a circuit.
- Current: The rate at which electrons flow. Measured in amperes (A).
- Capacity: How much charge a battery can store, usually measured in ampere-hours (Ah).
- Energy Density: How much energy a battery can store for its weight or volume. Important for portable devices and electric vehicles.
- Power Density: How quickly the battery can deliver energy.
Why Do Batteries Wear Out?
Batteries don’t last forever and for good reason. Each time you charge and discharge a battery, tiny changes occur in the materials inside.
For example:
- Electrode materials can degrade or lose structure.
- Electrolyte reactions can reduce overall efficiency.
Over time, this wear reduces the battery’s capacity and ability to hold a charge. That’s why your phone battery doesn’t last as long after a few years of use.
What Happens During Charging?
Charging a battery is like running the redox reaction in reverse.
- An external power source (like a wall charger) pushes electrons back into the anode.
- Ions also move in the opposite direction from the cathode to anode restoring the battery’s chemical potential.
- Once fully recharged, the battery is ready to go again.
This process must be carefully controlled. Overcharging or overheating can damage the battery or even cause it to catch fire, especially in high-energy batteries like lithium-ion.
What Makes Different Battery Types Unique?
Different batteries use different materials for their anodes, cathodes, and electrolytes. This changes how they perform. For example:
- Alkaline batteries (zinc and manganese dioxide) are cheap and stable but non-rechargeable.
- Lead-acid batteries are heavy but reliable commonly used in cars.
- Nickel-cadmium (NiCd) and Nickel-metal hydride (NiMH) are older rechargeable technologies.
- Lithium-ion batteries offer the best balance of energy density, cycle life and power but are more complex to manage.
New technologies like solid-state batteries and lithium-sulphur aim to push performance even further.
Why Does Temperature Matter?
Temperature has a big impact on battery performance. In cold temperatures:
- Ion movement slows down
- Internal resistance increases
- Capacity drops temporarily
In high heat:
- Reactions can accelerate uncontrollably
- Materials degrade faster
- Safety risks increase
This is why electric vehicles and advanced electronics often include thermal management systems to keep batteries within a safe temperature range.
Final Thoughts
Batteries are more than just containers for electricity they’re chemical engines. Through carefully balanced redox reactions, they transform stored chemical energy into the power that runs your world.
Whether it’s a button cell in a watch or a massive battery pack in a mining truck, the principles are the same: ions and electrons working in sync to deliver power when and where it’s needed.
As technology advances, batteries are getting more powerful, more reliable and more essential. Understanding how they work is the first step to appreciating just how much of our world depends on these silent powerhouses.